Lipids constitute a large part of the biomass of living organisms, and lipolytic enzymes play an essential role in the turnover of this material. In particular, triglycerides (triacylglycerols, TAGs) constitute the major part (95%) of dietary lipids. Human pancreatic (HPL) and gastric (HGL) lipases are essential enzymes for efficient fat digestion. The hydrolysis of dietary TAGs by these enzymes to monoacylglycerols and free fatty acids is a necessary step for fat absorption by the enterocytes. Therefore, potent and specific inhibitors of digestive lipases are of interest because they may find applications as anti-obesity agents. The β-lactone-containing inhibitor tetrahydrolipstatin is already used for the treatment of obesity (Orlistat/Xenical).
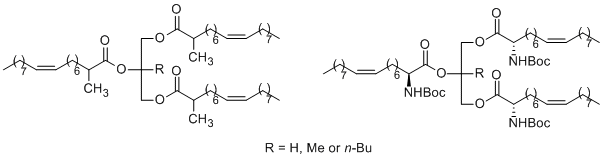
We have developed a novel class of potent human pancreatic and gastric lipase inhibitors. The sterically hindered triacylglycerol analogues are easily prepared and are very similar to the natural substrates of lipases from a structural point of view. Our results indicate that an alkyl group at the sn-2 position of the glycerol backbone or/and at the a-position of the acyl residue is an important substituent for the design and synthesis of potent inhibitors of digestive lipases, which might be useful for the inhibition of the hydrolysis and absorption of dietary fats. Furthermore, the triesters of glycerol and 2-methyl-glycerol with 2-(N-tert-butoxycarbonylamino)-oleic acid were also found to be potent inhibitors of HPL.

References
1. V. Magrioti, R. Verger, V. Constantinou-Kokotou, J. Med. Chem. 2004, 47, 288-291.
2. V. Constantinou-Kokotou, V. Magrioti, R. Verger, Chem. Eur. J. 2004, 10, 1133-1140.

