Natural products have served as a rich source for drug discovery and development. In the last decade their fruitful integration in the drug discovery pipeline declined due to their reduced bioavailability, mainly attributed to their poor aqueous solubility. We have investigated the interactions of the natural products quercetin (QUE) and silibinin (SLB) with (2-hydroxypropyl)-β-cyclodextrin (HP-β-CD). The complexation enabled amplification of the solubility of these natural products and in the same time retained their bioactivity. The stability of the complexes and the molecular basis of the interactions developed in this host-guest complex were assayed by incorporating an array of techniques such as: DSC, 2D DOSY NMR, solid and liquid high resolution NMR spectroscopy data, dissolution and solubility experiments, confocal microscopy, UV absorption and Molecular Dynamics (MD) computations.
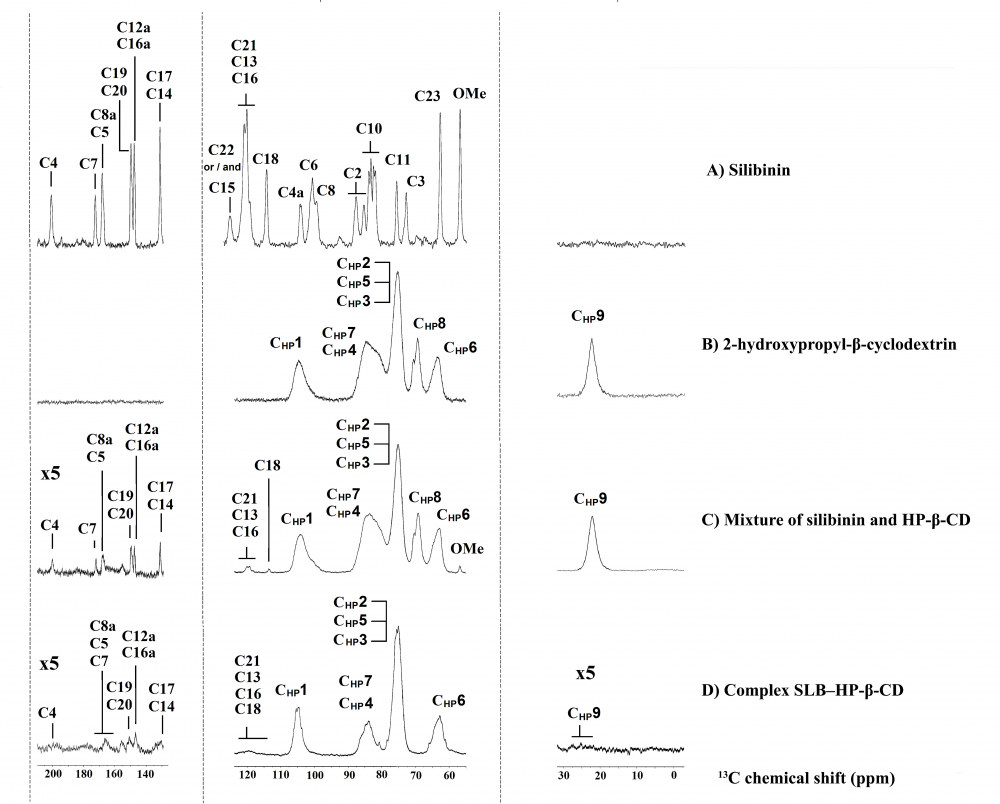
13C CP/MAS spectra of (A) silibinin, (B) HP-β-CD, (C) mixture of SLB‒HP-β-CD and (D) complex of SLB‒HP-β-CD.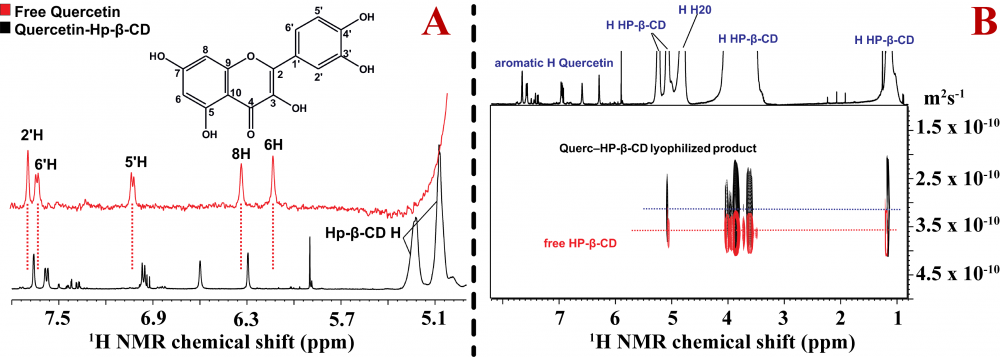
A) Overlapping of the 1H NMR of free quercetin colored in red (5.5mM) and complex of Quercetin–HP-β-CD (3.5 mM) diluted in D2O 0.78% DMSO-d6. B) Overlapping of the same spectral region from DOSY spectrum of 3.5 mM of QUE‒HP-β-CD lyophilized product, illustrated colored in black and free HP-β-CD (3.1 mM), colored in red. The spectra were recorded in D2O at 298K in 500 MHz.
In addition our group has explored the interactions of irbesartan (IRB) and irbesartan–2-hydroxypropyl-β-cyclodextrin (HP-β-CD) complex with dipalmitoyl phosphatidylcholine (DPPC) bilayers utilizing an array of biophysical techniques ranging from differential scanning calorimetry (DSC), small angle X-ray scattering (SAXS), ESI mass spectrometry (ESI-MS) and solid state nuclear magnetic resonance (ssNMR).
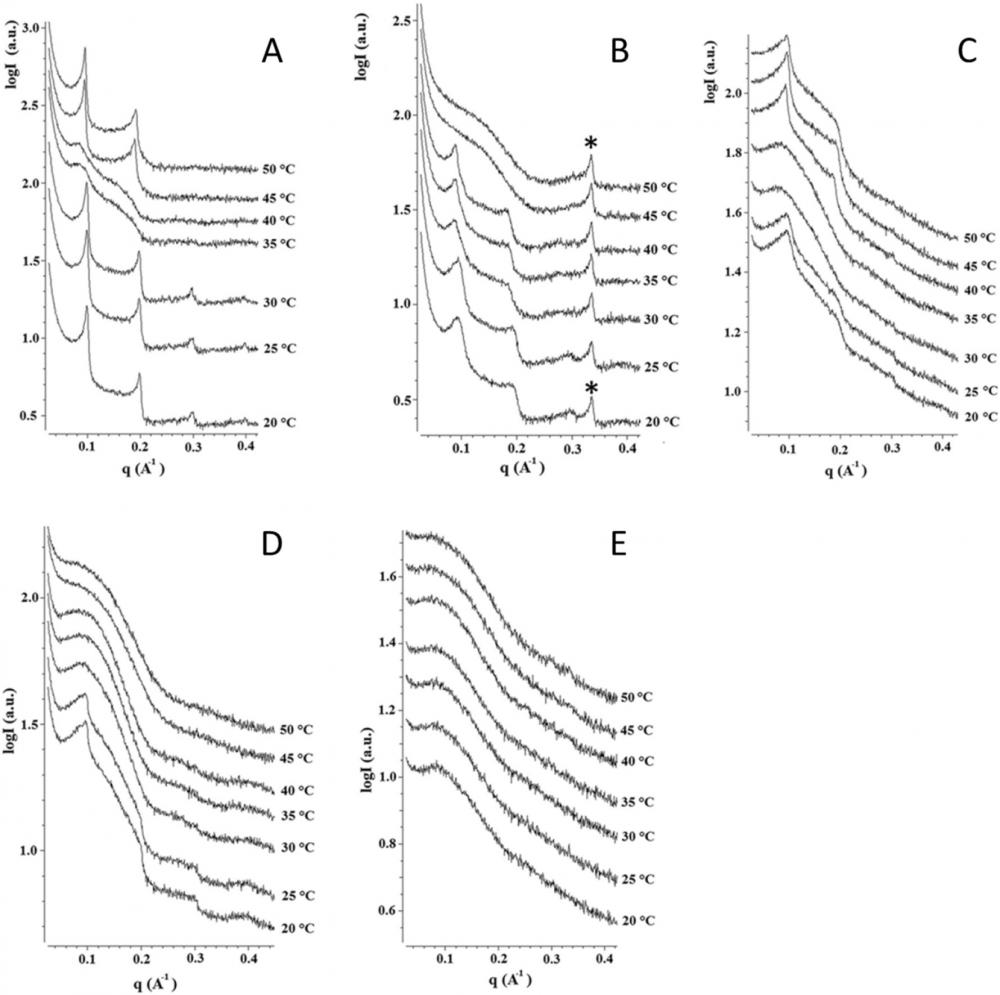
SΑΧS experiments on (Α) pure DPPC bilayers; (Β) DPPC/IRB bilayers (80:20); (C) DPPC/HP-β-CD bilayers (80:20); (D) DPPC MLV dispersion with [IRB/HP-β-CD] complex added (80:20); and (Ε) DPPC/[IRB/HP-β-CD] bilayers (80:20).
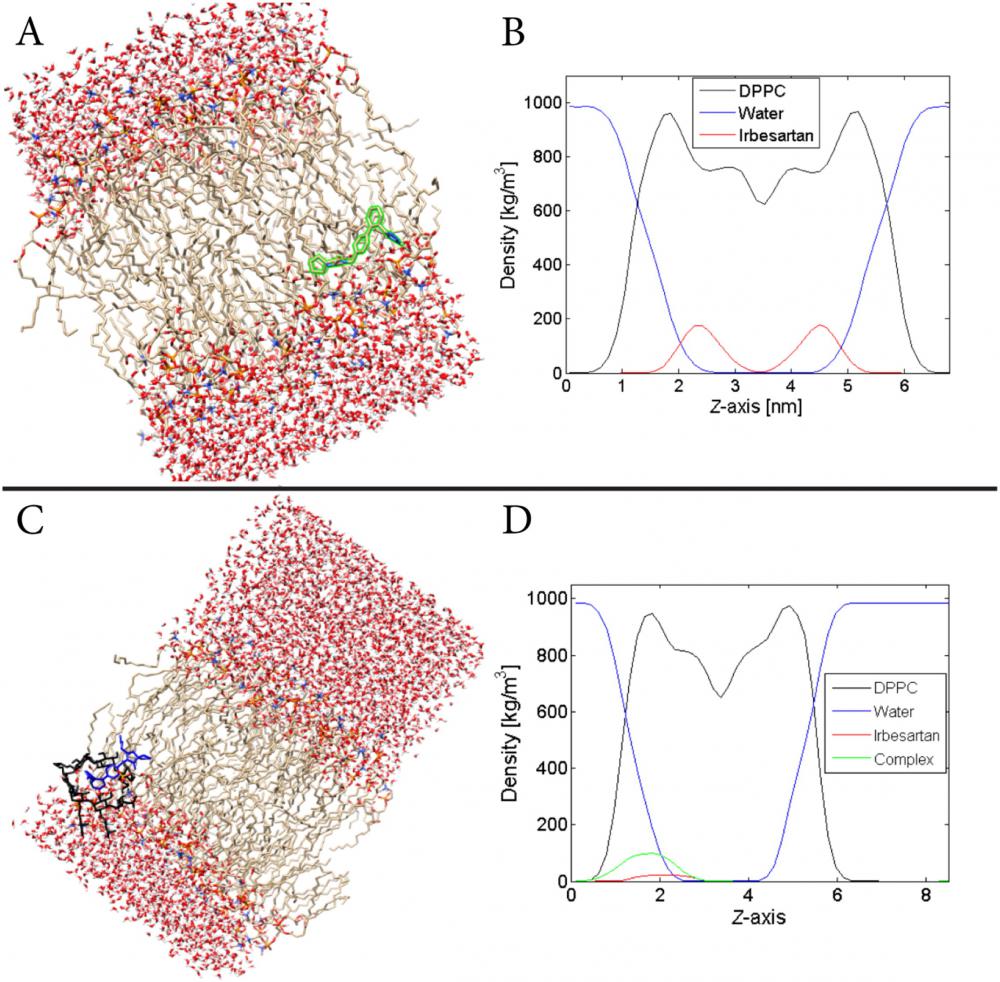
A) Snapshot of one IRB molecule in the DPPC bilayer. The green contour is employed for a better visualization of the molecule in question. B) Density profiles of water, DPPC and IRB molecules (12.2 mol%) along the Z-axis which is perpendicular to the two monolayers of the membrane. C) Graphical representation of IRB–HP-β-CD in the DPPC membrane in contact with an extended water phase. The IRB and CD derivative are depicted with blue and black colours, respectively. D) Density profiles of water, DPPC, IRB and IRB–HP-β-CD along the Z-axis during the last 0.25 μs of an MD simulation.
Relevant Publications
Professor Clemens Glaubitz (Institute for Biophysical Chemistry, Goethe Universität Frankfurt am Main)
Associate Professor G. Valsami (Faculty of Pharmacy, National and Kapodistrian University of Athens)
Assistant Professor A. G Tzakos (Faculty of Chemistry, University of Ioannina)
Assistant Professor M. Vlachou (Faculty of Pharmacy, National and Kapodistrian University of Athens)

13C CP/MAS spectra of (A) silibinin, (B) HP-β-CD, (C) mixture of SLB‒HP-β-CD and (D) complex of SLB‒HP-β-CD.

A) Overlapping of the 1H NMR of free quercetin colored in red (5.5mM) and complex of Quercetin–HP-β-CD (3.5 mM) diluted in D2O 0.78% DMSO-d6. B) Overlapping of the same spectral region from DOSY spectrum of 3.5 mM of QUE‒HP-β-CD lyophilized product, illustrated colored in black and free HP-β-CD (3.1 mM), colored in red. The spectra were recorded in D2O at 298K in 500 MHz.
In addition our group has explored the interactions of irbesartan (IRB) and irbesartan–2-hydroxypropyl-β-cyclodextrin (HP-β-CD) complex with dipalmitoyl phosphatidylcholine (DPPC) bilayers utilizing an array of biophysical techniques ranging from differential scanning calorimetry (DSC), small angle X-ray scattering (SAXS), ESI mass spectrometry (ESI-MS) and solid state nuclear magnetic resonance (ssNMR).

SΑΧS experiments on (Α) pure DPPC bilayers; (Β) DPPC/IRB bilayers (80:20); (C) DPPC/HP-β-CD bilayers (80:20); (D) DPPC MLV dispersion with [IRB/HP-β-CD] complex added (80:20); and (Ε) DPPC/[IRB/HP-β-CD] bilayers (80:20).

A) Snapshot of one IRB molecule in the DPPC bilayer. The green contour is employed for a better visualization of the molecule in question. B) Density profiles of water, DPPC and IRB molecules (12.2 mol%) along the Z-axis which is perpendicular to the two monolayers of the membrane. C) Graphical representation of IRB–HP-β-CD in the DPPC membrane in contact with an extended water phase. The IRB and CD derivative are depicted with blue and black colours, respectively. D) Density profiles of water, DPPC, IRB and IRB–HP-β-CD along the Z-axis during the last 0.25 μs of an MD simulation.
Relevant Publications
- Kellici, T. F.; Ntountaniotis, D.; Leonis, G.; Chatziathanasiadou, M.; Chatzikonstantinou, A. V.; Becker-Baldus, J.; Glaubitz, C.; Tzakos, A. G.*; Viras, K.; Chatzigeorgiou, P.; Tzimas, S.; Kefala, E.; Valsami, G.*; Archontaki, H.; Papadopoulos, M. G.; Mavromoustakos, T.*, Investigation of the interactions of silibinin with 2-hydroxypropyl-β-cyclodextrin through biophysical techniques and computational methods. Molecular Pharmaceutics 2015, 12, 954-965.
- Kellici, T. F.; Chatziathanasiadou, M. V.; Diamantis, D.; Chatzikonstantinou, A. V.; Andreadelis, I.; Christodoulou, E.; Valsami, G.; Mavromoustakos, T.; Tzakos, A. G.*, Mapping the interactions and bioactivity of quercetin—(2-hydroxypropyl)-β-cyclodextrin complex. International Journal of Pharmaceutics 2016, 511, 303-311.
- Liossi Α. S., Ntountaniotis D., Kellici T. F., Chatziathanasiadou M. V., Μegariotis G., Mania M., Becker-Baldus J., Kriechbaum M., Krajnc A., Christodoulou E., Glaubitz C., Rappolt M., Amenitsch H., Mali G., Theodorou D. N., Valsami G., Pitsikalis M., Iatrou H., Tzakos A. G., Mavromoustakos T.*, Exploring the interactions of irbesartan and irbesartan–2-hydroxypropyl-β-cyclodextrin complex with model membranes. BBA-Biomembranes, 2017, 1859, 1089-1098
- Vlachou, M.*; Siamidi A.; Diamantidi E.; Iliopoulou A.; Ioannidou V.; Kourbeli V.; Foscolos A-S.; Papanastasiou I.; Vocat A.; Cole S.T.; Karalis V.; Kellici T.; Mavromoustakos T., In vitro Controlled Release from Solid Pharmaceutical Formulations of two new Adamantane Aminoethers with Antitubercular Activity (I). Drug Research; Thieme, 2017, 47, 447-450
- Vlachou M.*, Siamidi A., Spaneas D., Lentzos D., Ladia P., Anastasiou K., Papanastasiou I., Foscolos A.-S., Georgiadis M.-O., Karalis V., Kellici T., Mavromoustakos T. In vitro Controlled Release of two new Tuberculocidal Adamantane Aminoethers from Solid Pharmaceutical Formulations (II), Drug Research; Thieme, DOI: 10.1055/s-0043-114012
Professor Clemens Glaubitz (Institute for Biophysical Chemistry, Goethe Universität Frankfurt am Main)
Associate Professor G. Valsami (Faculty of Pharmacy, National and Kapodistrian University of Athens)
Assistant Professor A. G Tzakos (Faculty of Chemistry, University of Ioannina)
Assistant Professor M. Vlachou (Faculty of Pharmacy, National and Kapodistrian University of Athens)

