Genetic and environmental factors interact for the determination of phenotype, both normal and pathological. Despite this fact, in most cases, animal models of diseases employ only genetic interventions (Crispr technology, knock-out or transgenes) to address the etiopathogenesis of human conditions with documented genetic bases, which account to a small percent of all human diseases. Few animal disease models employ environmental manipulations, mostly to simulate psychiatric conditions. In these models, negative early experiences have been used, usually in the form of disruption of mother-offspring interactions, to investigate the underlying mechanisms of conditions such as depression, anxiety and prefrontal cortex-dependent deficits. In the present study an early experience of mild adversity, which has been documented to model child neglect, will be employed to investigate prefrontal cortex development and function in the rat.
WP2 Role of dopamine in neuronal proliferation and differentiation during the early postnatal development
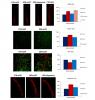
The DER experience-induced dopamine depletion, prohibited the normal maturation of dividing cells in the lateral ventricles of the brain, halting dividing cells in early stages of differentiation, and eventually leading them to apoptosis.
WP3 Role of dopamine in neuronal migration during the early postnatal development
Depletion of dopamine due to the DER experience possibly induces aberrant migration trajectory, resulting in reduced number of neurons in the medial orbital prefrontal cortex.
WP4 Type of dopamine receptors responsible for the developmental effects of dopamine
Short-term effects:
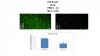
The DER-induced effects on neurons and glial cells in the PFC on PND13 can be mimicked by administration of D1-like or D2-like antagonists for neurons and glial cells, respectively. It seems that dopamine can influence, at least during development, neuronal and glial populations through distinct, non-overlapping types of dopamine receptors.
Long-term effects:
The DER-induced effects on PFC-mediated behaviours in adulthood can be mimicked by administration of D1-like or D2-like antagonists during the neonatal period (PND10-13). Whereas, the DER-induced effects on neurons in the PFC in adulthood can be mimicked by administration of D1-like antagonist, but not D2-like antagonist, during the neonatal period (PND10-PND13).
WP5 Short- and long-term effects of glial activation during the early postnatal development
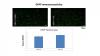
The DER-induced effects on astrocytes and neurons in the ventro-medial part of the PND13 or adult prefrontal cortex, in general, are not dependent on the DER-induced microglia activation.
More specifically, deactivation of microglia with the use of a specific inhibitor (PLX5622) during the period of DER training, generally does not have any impact on the DER-induced cellular effects in the medial orbital PFC or the DER-induced hypofrontality as indicated by deficits in PFC-mediated behavioral tasks or the DER-induced anhedonia. The sole exception in this lack of effects being a normalization of spine densities on MO glutamatergic neurons in adulthood.
WP6 Involvement of microglia on the DER phenotype on prefrontal cortex
Activation of microglia with the use of a specific activator (T0070907) during PND10-13 (the same period as that of DER training), does not induce any cellular effects in the PFC or any behavioral deficit in PFC-dependent tasks.
WP7 Type of dopamine receptors responsible for the effects of PFC dopamine depletion on micro- and astroglia
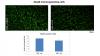
Depletion of dopamine due to the DER experience induces micro- and astroglia activation in PND13 medial orbital prefrontal cortex. This phenomenon is prevented by activation of D2-like receptors.
aberrant migration trajectory, resulting in reduced number of neurons in the medial orbital prefrontal cortex.