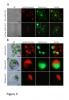
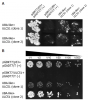
Ubiquitin mediated protein degradation constitutes one of the most complex post translational gene regulation mechanisms in eukaryotes. This fine-tuned proteolytic machinery is based on a vast number of E3 ubiquitin ligase complexes that mark target proteins with ubiquitin. The specificity is accomplished by a number of adaptor proteins that contain functional binding domains, including the WD40 repeat motif (WDRs). To date, only few of these proteins have been identified in plants. An RNAi‑mediated silencing approach was used here to functionally characterize the Arabidopsis thaliana ULCS1 gene, which encodes for a small molecular weight WDR protein. AtULCS1 interacts with the E3 Cullin Ring Ligase subunit DDB1a, regulating most likely the degradation of specific proteins involved in the manifestation of diverse developmental events. Silencing of AtULCS1 results in sterile plants with pleiotropic phenotypes. Detailed analysis revealed that infertility is the outcome of anther indehiscence, which in turn is due to the impairment of the plants to accomplish secondary wall modifications. Furthermore, IREGULAR XYLEM gene expression and lignification is diminished in anther endothecium and the stem vascular tissue of the silenced plants. These data underline the importance of AtULCS1 in plant development and reproduction.
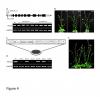
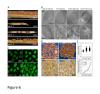
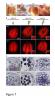
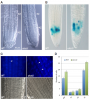
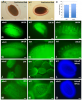
RNAi-mediated downregulation of ULCS1 results in a fast-growing phenotype during vegetative development. Due to accelerated growth, ulcs1i mutants reach their vegetative to reproductive transition point earlier than WT plants. However, their comparable germination rate and their similar number of secondary branches and rosette leaves at bolting indicate that ulcs1i is not an early flowering time mutant. GUS staining of progeny, obtained from crosses between ulcs1i and CYCB1::GUS plants, revealed an increased number of mitotic cell divisions in the root meristems of ulcs1i compared to WT. Immunolabeling of homogalacturonans (HGAs) epitopes showed significant fluorescent signal differences at the cell walls and the mucilage of the seeds between ulcs1i and WT. Furthermore, we demonstrate that ULCS1 interacts with the UBA-like protein in a yeast two-hybrid assay, suggesting a direct or indirect physical coupling of these proteins in Arabidopsis.

| Beris et al., 2016.pdf | 5.33 MB | |
| Beris et al., 2022.pdf | 2.65 MB |