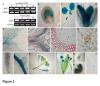
Arabidopsis thaliana flowering time mutants revealed the function of numerous genes that regulate the transition from vegetative to reproductive growth. Analyses of their loci have shown that many of them act as chromatin modifiers. WD40-repeat proteins (WDRs) are present in all eukaryotes and include members that are implicated in numerous cellular activities. They act as scaffold proteins and thus as molecular "hubs" for protein-protein interactions, which mediate the assembly of multifunctional complexes that regulate key developmental processes in Arabidopsis thaliana, such as flowering time, hormonal signaling, and stress responses. Despite their importance, many aspects of their putative functions have not been elucidated yet.
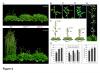
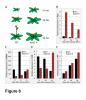
A combination of molecular and genetic approaches have been implemented, to characterize the function of APRF1 (ANTHESIS PROMOTING FACTOR 1) gene in A. thaliana and to investigate its role in plant development. APRF1 encodes for a low molecular weight nuclear WDR protein which displays functional homology to the Swd2 protein, an essential subunit of the yeast histone methylation COMPASS complex. Compared to WT plants, total loss‑of‑function aprf1 mutants exhibited shoot apical meristem (SAM) alterations and increased growth rates. However, the vegetative phase of aprf1 plants was prolonged and bolting was delayed, indicating an impairment in flowering under long days (LD). On the contrary, overexpression of APRF1 accelerates flowering. Consistent with the late flowering phenotype, the molecular data confirmed that FLC and SOC1 expression were significantly altered in the aprf1 mutants. Our data suggest that APRF1 acts upstream of FLC and promotes flowering under LD.
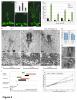
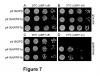
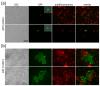
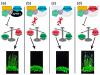
Moreover, the late-flowering phenotype of the anthesis promoting factor 1 (aprf1) mutants is temperature dependent and can be suppressed when plants are grown under mild heat stress conditions. To gain further insight into the mechanism of APRF1 function, we employed a Co Immunoprecipitation (Co-IP) approach to identify its interaction partners. We provide the first interactome of APRF1, which includes proteins that are localized at several subcellular compartments and are implicated in diverse cellular functions. The dual nu-cleocytoplasmic localization of ARRF1, which was validated through the interaction of APRF1 with HEAT SHOCK PROTEIN 1 (HSP90.1) in the nucleus and with HSP90.2 in the cytoplasm, in-dicates a dynamic and versatile involvement of APRF1 in multiple biological processes. The specific interaction of APRF1 with chaperon HSP90.1 in the nucleus expands our knowledge re-garding the epigenetic regulation of flowering time in A. thaliana and further suggests the existence of a delicate thermoregulated mechanism during anthesis.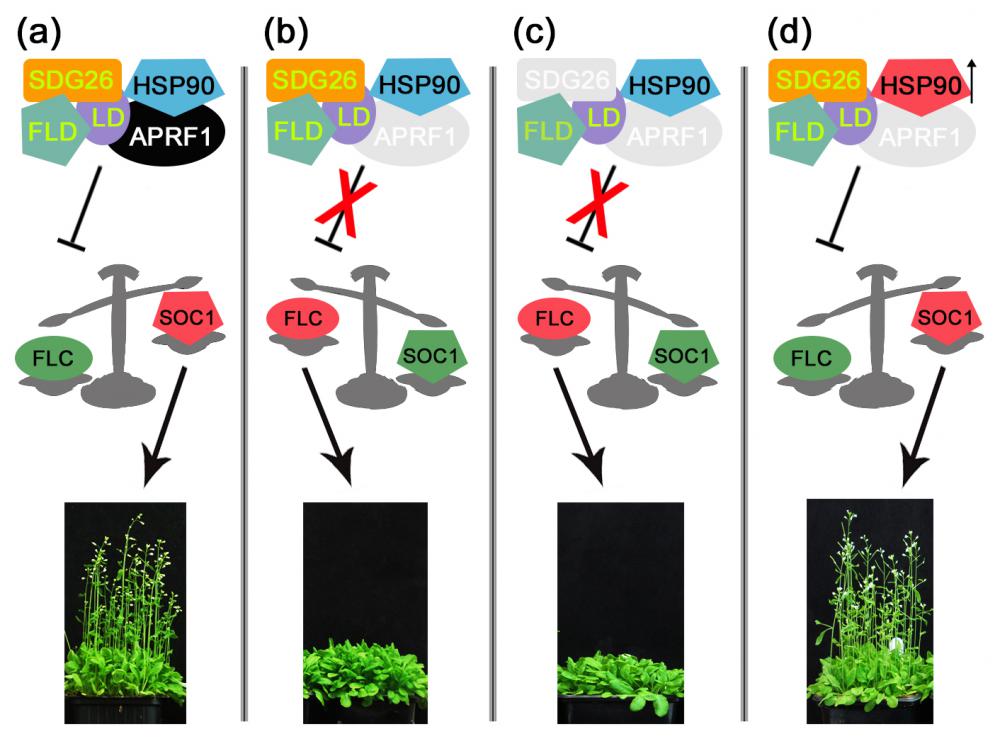
| Haralampidis et al., 2002.pdf | 678 KB | |
| Prassinos et al., 2008.pdf | 551 KB | |
| Kapolas et al., 2016.pdf | 5.9 MB | |
| Samakovli et al., 2022.pdf | 5.24 MB | |
| Plitsi et al., 2022.pdf | 4.82 MB | |
| Ιsaioglou et al., 2024.pdf | 5.18 MB |